We are a multi-disciplinary research group focused on developing quantitative biomarkers for non-invasive, real-time assessments of tissue structure and function to diagnose disease or trauma and guide therapies.
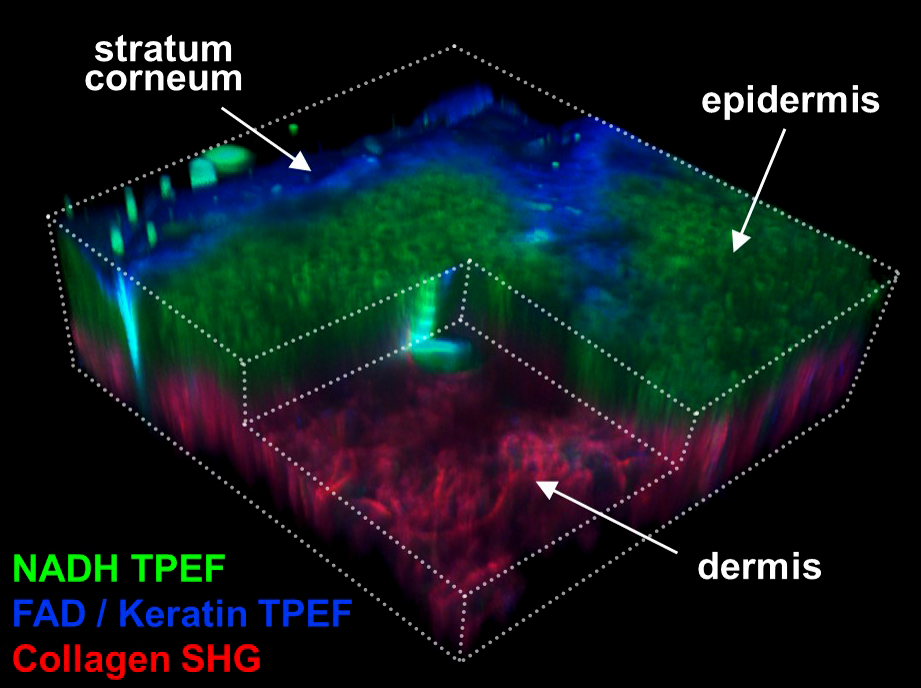
Label-free multiphoton microscopy can provide a variety of non-invasive quantitative biomarkers of cell metabolism, collagen fiber organization, and tissue composition. By quantifying the naturally present fluorescence of NADH and FAD without our cells, we can non-invasively measure dynamic changes in the metabolism of live cells, tissues, and organisms. Second harmonic generation (SHG) imaging can also provide a non-destructive measure of 3D collagen organization within tissues. While acquiring two-photon excited fluorescence (TPEF) and SHG signals, we can also measure coherent anti-Stokes Raman scattering (CARS), which provides images of a tissue’s protein and lipid composition. We combine these imaging methods with deep learning and advanced image analysis approaches to quantify the orientation, morphology, and fractal organization of different cell and tissue features.
The following selected press releases provide an overview of our techniques and how we are using them to solve various biomedical research and health-related problems: